CCD Issues Notice of Rule Hearing and Proposed Rulemaking for September 3, 2025: What Stands Out…
The New Mexico CCD has dropped its latest round of proposed rule changes, and they aren’t small tweaks. At the heart of it all is a brand-new rule — 16.8.9 NMAC — that lays out how the state can embargo, recall, and seize product. It’s a sweeping enforcement framework: mandatory recalls, destruction procedures, tagging and surveillance requirements, penalties up to $10,000 per violation, and even coordination with law enforcement for seizures. Think FDA recall power, but applied to New Mexico’s market.
Beyond that, amendments ripple across the rulebook. Exit packaging is out (16.8.3.13 NMAC). Retail deliveries are narrowed, limited strictly to residential addresses by licensed couriers, who may carry no more than $5,000 worth of product (16.8.2.40 NMAC and 16.8.2.41 NMAC). Testing standards are tougher: minimum sample sizes are raised and pesticide testing exemptions are eliminated (16.8.2.48 NMAC and 16.8.7.15 NMAC). Labels will need more information — retail license numbers, testing lab info, test dates, strain lineages, and even mother plant COAs (16.8.3.9 NMAC and 16.8.3.10 NMAC). Microbusinesses get a modest win in the form of licensing fee discounts tied to consignment deals with retailers (16.8.11.15 NMAC). And while personnel recordkeeping requirements expand (16.8.2.20 NMAC), the burdensome biennial audit requirement is scrapped (also 16.8.2.20 NMAC).
More Amendments…
The CCDs latest proposed amendments touch nearly every corner of the rulebook, some are just cleanup, but a few sections stand out for how they could alter the day-to-day operations of retailers, lounges, and product teams. On paper, these changes look small. In practice, they carry weight.
Defining Deli-Style Sales
The Division has added a formal definition of deli-style manufacturing/retail: product portioned, packaged, or weighed at the time of sale rather than sold pre-packaged. Staff must meet the same standards for packaging, labeling, and sanitation as manufacturers.
Speculation: Deli-style flower has already been active in New Mexico. Adding the definition is more about tightening the rulebook than changing the reality on the ground. No big operational shift here, just more clarity for inspectors.
16.8.2.40 – Retail Standards
The amendment removes the prohibition on selling or consuming products that have been removed from packaging for display.
Speculation: This is a win for retailers. Being able to display concentrates, flower, or edibles without regulatory fear makes merchandising easier and potentially cuts costs. For consumers, though, the picture is mixed: are you buying a pristine product or one that’s been opened and sniffed by five different people? The rule opens the door to better sales presentation but also raises a question about consumer trust.
16.8.2.49 – Consumption Area Licensure
The rule limits consumption lounges to selling only pre-packaged 10 mg or less units, purchased on site.
Speculation: On one hand, this makes sense — most lounges don’t push high-dose servings anyway, and from a consumer safety standpoint, 10 mg is a logical ceiling. On the other hand, the way this reads suggests lounges can only offer pre-packaged 10 mg products. The issue? Not a lot of brands actually produce single-serving 10 mg packages. Does that mean a retailer could open a 100 mg edible and repackage it into 10 mg units? That’s unclear, and it’s the kind of vagueness that lounge operators will want to watch closely.
16.8.2.54 – Consumption Area Standards
Lounges will need to maintain a copy of a passing Certificate of Analysis (COA) for every product available.
Speculation: Hard to argue with this one — it’s a clear consumer health safeguard. The burden lands on operators, who now need a system to manage, store, and show COAs at the dab bar. It’s another layer of paperwork, but not an unreasonable one.
16.8.3.9 – Finished Product Labeling
Labels must now include:
The license number of the retail licensee that sold the product.
The name and license number of the testing lab.
The date of the finished product test.
Speculation: This is where headaches start for product people. Pre-printed labels and branded packaging are suddenly outdated. Every box, bag, and jar will need additional details that can vary from batch to batch. That means either ordering more customizable packaging, or applying supplemental stickers. Forget to track this data properly, and you’ll burn through labels — and cash — trying to keep compliant.
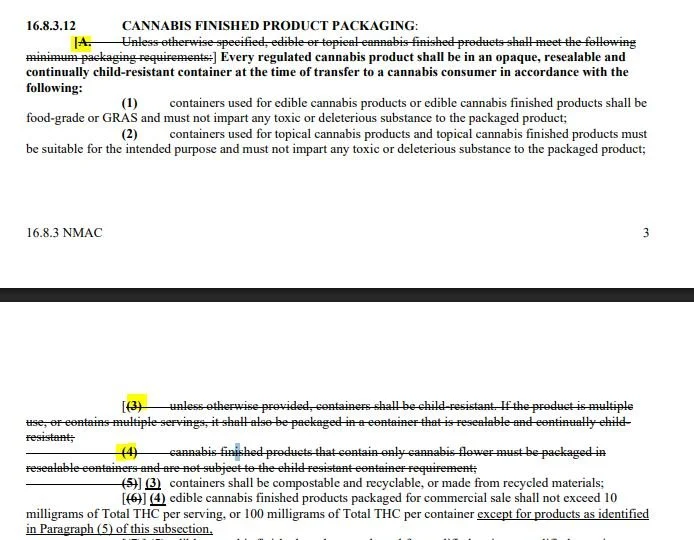
16.8.3.12 – Packaging Standards
Requires every regulated product — potentially including flower — to be in an opaque, resealable, child-resistant container at the time of transfer.
Section 16.8.3.12 (A) - crosses out “unless otherwise specified” Language, as well as A (4), which references Flower not being subject to CR packaging.
Speculation: This could be a costly change. Currently, flower often ships in turkey bags, vac-sealed packs, or terp-lock bags with windows so buyers can see the product. Under the proposed rules, visibility is out, and every transfer must be in a child-resistant package. It tightens safety optics but ignores a real-world quality control point: consumers and retailers often want to see what they’re buying.
Public Voice
On paper, some of this reads like cleanup: clarifying cross-references, cutting outdated provisions, or eliminating redundancy. But in practice, it’s a whole new layer of compliance, with the potential to slow production, choke margins, and squeeze the smallest operators hardest.
That’s why the public comments matter. The Division posts every submission online, and the early ones highlight the stakes perfectly. A prominent business owner warned that eliminating testing exemptions for infused products will create redundant, costly bottlenecks that New Mexico’s limited lab infrastructure cannot handle. The math is simple: what’s now a 3–5 day production cycle could stretch to 14–21 days, leaving shelves empty, patients waiting, and capital frozen in inventory. Other states have tried similar rules and paid the price — California in 2018, Michigan soon after, Oregon eventually forced to reverse course. The lesson is clear: over-testing doesn’t equal safer product, it just means more delays and higher costs.
The Operators letter is 100% accurate. This is exactly the kind of practical pushback regulators need to hear. Because while the CCD is tasked with protecting consumers, they’re writing rules in a vacuum. What the industry needs right now is to set differences aside — small grower or MSO, manufacturer or retailer — and speak with one voice. Not to fight safety standards, but to help the CCD understand how this world actually works before they change it.
At the end of the day, no one can take care of you and your business better than you, be aware.
More Information…
Public Rule Hearing
Date: September 3, 2025
Time: 9:00 AM
Location: Rio Grande Conference Room, Toney Anaya State Office Building, 2550 Cerrillos Road, Santa Fe, NM
Purpose
To receive public comment on proposed rule changes.
Includes creation of a new rule (16.8.9 NMAC) covering Embargo, Recall, and Seizure of Cannabis.
Also includes amendments to various existing rules affecting all license types.
Public Comment Period
Opens: July 29, 2025
Closes: At the conclusion of the hearing on September 3, 2025
Ways to Submit Comments
Email: ccd.publiccomment@state.nm.us
Mail:
Cannabis Control Division Public Comment
c/o Bradford A. Borman
P.O. Box 25101
Santa Fe, NM 87504Online: CCD Rulemaking Page
All comments received before the hearing will be posted on the CCD website.
Access to Full Notice
A full copy of the proposed rules, rule hearing notice, and a summary of changes is available at:
https://www.rld.nm.gov/cannabis/laws-rules-regulations/rulemaking/
Proposed Amendments to Existing Rules (2025 CCD Rulemaking)
16.8.1 – General Provisions
New Definitions Added:
Audited product, deli-style, flowering, inhaled product, mature plant, oral consumption, representative sample, skin & body products.
Advisory Committee Meetings: May be held remotely at Superintendent’s discretion.
16.8.2 – Cannabis Establishments
16.8.2.8 General Operations
Ban on free non-medical product moved from 16.8.2.40.
Removes prior recall rules (now handled by new 16.8.9).
Chain of custody documentation updated.
16.8.2.13 Transportation
Clarifies shipping manifest requirements.
16.8.2.20 Monitoring
Must keep personnel records for 2 years post-separation (employees + contractors).
Eliminates biennial audit submission requirement.
16.8.2.21 Producer Licensure
Fixes incorrect cross-reference.
16.8.2.22 Producer License Application
Must provide proof of lawful possession of premises (ownership or owner acknowledgment).
16.8.2.27 Production
Fixes cannabis waste rule citation.
16.8.2.29 Manufacturing – General
Clarifies activities limited by license type.
Restricts all unlicensed manufacturing activity.
Updates prohibited additives, with special rules for oral consumption products.
16.8.2.30 Manufacturer License Application
Removes requirement for NMED approval for topicals.
Adds proof of lawful possession of premises requirement.
16.8.2.34 Manufacturing Standards
Adds rules for deli-style transactions at co-located retail/manufacturing sites.
Clarifies conversion restrictions.
16.8.2.36 Retailer License Application
Adds proof of lawful possession requirement.
16.8.2.40 Retail Standards
Removes ban on display of unpackaged products.
Moves prohibition on free non-medical cannabis to 16.8.2.11.
Clarifies delivery: only licensed couriers, to residential addresses only.
16.8.2.41 Courier
Payments: any legal method (including gift card pre-pay), but excludes EBT cards.
Reduces max vehicle value to $5,000.
Adds clarity on delivery intervals.
16.8.2.43 Testing Labs – General
Prevents individuals with an interest in cannabis businesses from owning or working in labs.
16.8.2.44 Testing Lab Application
Adds proof of lawful possession requirement.
16.8.2.45 Amended Lab License Application
Defines “material/substantial modifications” (size, sale/purchase of property, relocation).
Requires CCD approval for changes in testing methods (limited to once per year at renewal).
Requires demonstration of capability for new methods.
16.8.2.48 Testing Standards
Raises minimum sample sizes:
Microbial: ≥1g; Non-microbial: ≥0.5g.
Dried cannabis sampling adjusted by batch weight.
Edibles/topicals/concentrates/volatile solvent batches scaled by weight.
Removes option to retain non-destroyed test portions for “internal control.”
16.8.2.49 Consumption Areas – General
Products limited to pre-packaged 10mg or less units, purchased onsite.
16.8.2.54 Consumption Standards
Must maintain a passing COA on all products available for consumption.
16.8.3 – Labeling & Packaging
16.8.3.9 Labeling – Finished Products
Must include:
Retail licensee number.
Testing lab name + license number.
Date of finished product test.
16.8.3.10 Labeling – Seeds & Immature Plants
Must include producer license number, strain names (including parents), germination rate, THC thresholds.
Requires mother plant COA at sale.
16.8.3.12 Packaging – Finished Products
Every product must be in opaque, resealable, child-resistant packaging at transfer.
Medical exception: may exceed 10mg serving / 800mg package limits.
Removes “single-serving only” for liquids.
Removes transitional rules for pre-CRA medical packaging.
16.8.3.13 Exit Packaging
Eliminates requirement entirely.
16.8.7 – Testing
16.8.7.15 Required Testing
Updates testing table & potency re-test limits.
Removes pesticide test exemption for products made from previously tested concentrates.
Allows only one re-test by any licensed lab.
Requires remediation labels: “Remediated.”
Any pesticide fail = mandatory destruction.
16.8.8 – Plant Limits
16.8.8.9 Plant Tiers
Removes authority for licensees to increase plant counts by 8 increments.
16.8.8.10 Plant Increase Requests
Removes factors tied to medical sales minimums or shortages.
16.8.11 – Fees
16.8.11.8 General Fee Provisions
All payments must come from authorized sources.
Unauthorized payments = incomplete/expired applications.
Effective Jan 1, 2027.
16.8.11.15 Retailer Fee Discount
Establishes discount eligibility for consignment contracts between retailers & microbusinesses.
Big Picture Takeaways
Stricter compliance on product safety: Testing, labeling, packaging, and recall authority all tightened.
Loosening in some areas: Exit packaging eliminated; display rules softened.
Administrative relief: Biennial audit requirement removed, but offset by stronger personnel recordkeeping.
Retail & delivery tightened: Couriers capped at $5K in product value, residential-only delivery, explicit bans on EBT.
Microbusiness support: New fee discounts for consignment partnerships.
Lab independence reinforced: No cross-ownership with other licensees.
16.8.9 NMAC – Embargo, Recall, and Seizure of Cannabis
Effective Date: July 1, 2025 (unless noted otherwise)
Authority: Cannabis Regulation Act & Lynn and Erin Compassionate Use Act
Sections Overview
16.8.9.1 – 16.8.9.7
Scope & Authority: Applies to all licensees; gives CCD authority over embargoes, recalls, and seizures.
Objective: Protect public health, ensure regulatory compliance.
Definitions: Adopted from CRA, Lynn & Erin, and Part 1 NMAC.
16.8.9.8 – General Provisions
Cooperation: Licensees must comply with CCD investigators (no obstruction, intimidation, denial of access, or false info).
Voluntary Recalls:
Must have written procedures.
Must cover when, how, who, and notification steps (customers, supply chain, media).
Destroy recalled products at licensee’s expense.
Notify CCD within 24 hours of initiating recall.
Penalty mitigation if recall initiated within 48 hours of identifying an issue.
16.8.9.9 – Embargo Procedures
Grounds: If products are evidence of violations or threats to public safety.
Notice: Emailed to licensee + posted online (valid if not bounced within 24 hrs).
Duration: Only as long as needed for investigation or corrective action.
Tagging/Storage:
Must be visibly tagged: “NOT FOR SALE OR DISTRIBUTION – UNDER EMBARGO.”
Stored in secure, limited-access area.
Restrictions: Cannot move, sell, or alter products without CCD approval.
Tracking: Must maintain full audit trail in BioTrack.
Inspections: CCD can check at any time.
Temporary Movement: Only for lab testing with written CCD approval.
Corrective Relabeling: Allowed if only labeling/packaging issue (with CCD sign-off).
16.8.9.10 – Recall Orders
CCD Authority: May recall products if adulterated, dangerously misbranded, illicit, or a health/safety threat.
Notice: Email + website posting with product details, basis, instructions, and timeline.
Corrective Action: Relabel/repackage allowed only if no safety risk.
Licensee Duties:
Stop all sales immediately.
Notify customers & downstream licensees.
Tag & secure product.
File recall report within 48 hours (quantities, evidence of destruction, corrective actions).
16.8.9.11 – Notice and Hearing
Right to Hearing: Licensees can appeal embargo, seizure, or recall.
Request Deadline: Within 10 days of notice.
Hearing Timeline: Must be scheduled within 30 days.
Burden of Proof: CCD must prove by preponderance of evidence.
Final Decision: Superintendent issues within 15 business days of recommendation.
Judicial Review: Allowed under NM law.
16.8.9.12 – Division Actions Following Seizure
Coordination: CCD works with Enforcement Bureau; must issue written notice to licensee.
Licensee Duties:
Preserve all related records.
Maintain secure storage.
Provide CCD access.
No movement, tampering, or destruction without approval.
Further Actions: May lead to embargo, recall, Notice of Contemplated Action, condemnation petition, or other enforcement.
16.8.9.13 – Condemnation and Destruction
CCD Petition: If product is deemed illegal/adulterated/misbranded.
Licensee Options: Has 5 days to destroy at their own expense or CCD files petition in district court.
Court Order: If granted, destruction is supervised by CCD.
Costs: Licensee bears all destruction expenses.
If Denied: Product released back to inventory.
Records: Licensees must keep all embargo/seizure/condemnation records for 2 years.
16.8.9.14 – Cooperation with Other Agencies
CCD may coordinate with Enforcement Bureau, Dept. of Ag, Dept. of Environment, and others as needed.
16.8.9.15 – Penalties
Criminal: Knowingly removing, concealing, or selling embargoed/recalled products may lead to criminal charges (NM §31-18-15).
Civil: CCD may impose fines up to $10,000 per violation, suspend/revoke licenses, or pursue additional administrative action.
16.8.9.16 – Severability
If part of the rule is struck down, the rest remains enforceable.
Key Takeaways
This rule essentially codifies a full enforcement framework for embargoes, recalls, seizures, and destruction of cannabis products.
It mirrors FDA-style recall/embargo authority but tailored to the cannabis market.
Licensees are on the hook for costs of recalls, destruction, and recordkeeping.
Due process protections exist, but CCD’s authority is broad and immediate.
Mitigation incentives (like voluntary recalls within 48 hours) show CCD is trying to balance enforcement with cooperation.